What does it take to turn a botanical into an FDA-Approved drug?
The FDA has a unique, streamlined process for medicinally active plant-based products to gain market authorization. That this process is much faster...
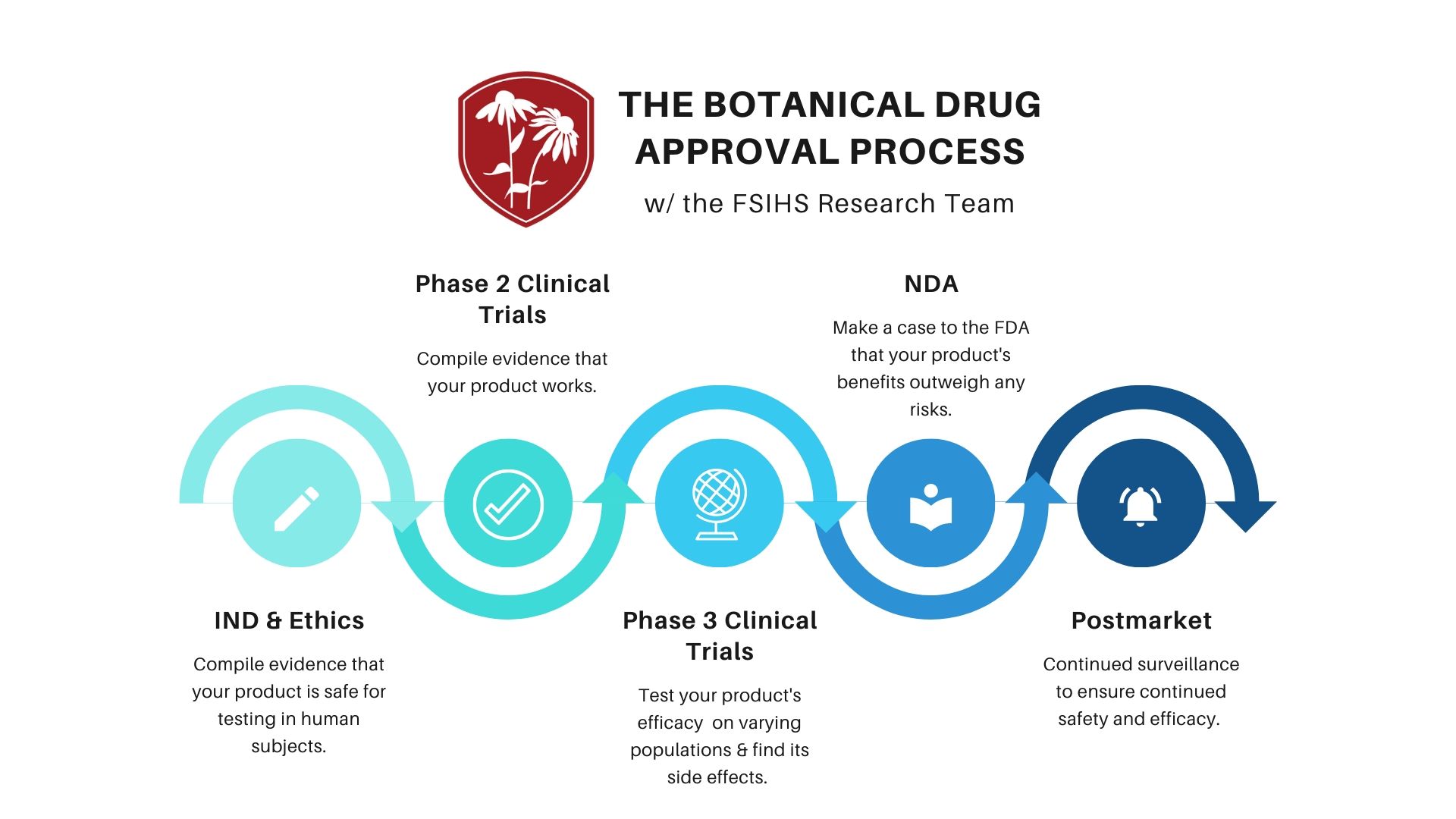
Natural products created from plant-based ingredients are often capable of powerful medicinal effects in the human body. Turning these formulations into an FDA-authorized drug is actually much easier and faster (and far less expensive) than many biotechnology companies realize.
Most botanical products begin with an IND application and are able to submit evidence of extensive human experience with the product in place of completing steps 2 and 4.
Some CROs provide simple cookie-cutter packages with general statistical reports. Our complete packages are developed with your end goal in mind, providing you user-friendly reports, submission ready documentation, and management of regulatory processes, where applicable.
Our team even compiles a research overview in lay terms for delivery to your entire team. Your sales department will love this user-friendly approach.

The FDA has a unique, streamlined process for medicinally active plant-based products to gain market authorization. That this process is much faster...
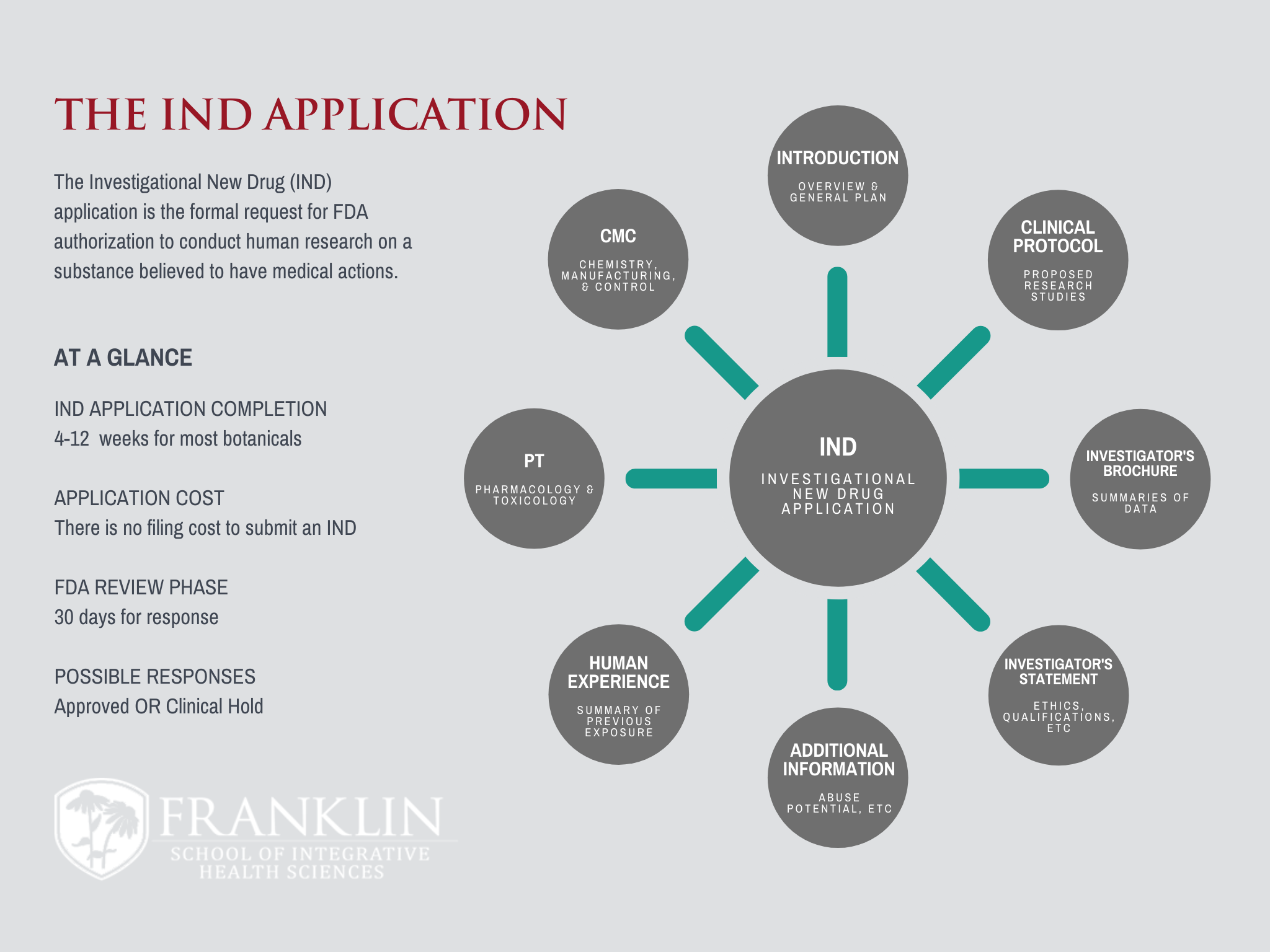
If your study involves a test drug and a medical outcome (i.e. something to prevent, diagnose, or treat disease), the FDA requires an Investigational...

Clinical trials have a reputation for being massive expenditures, with budgets easily running to the millions. While this may be the case for certain...