What does it take to turn a botanical into an FDA-Approved drug?
The FDA has a unique, streamlined process for medicinally active plant-based products to gain market authorization. That this process is much faster...
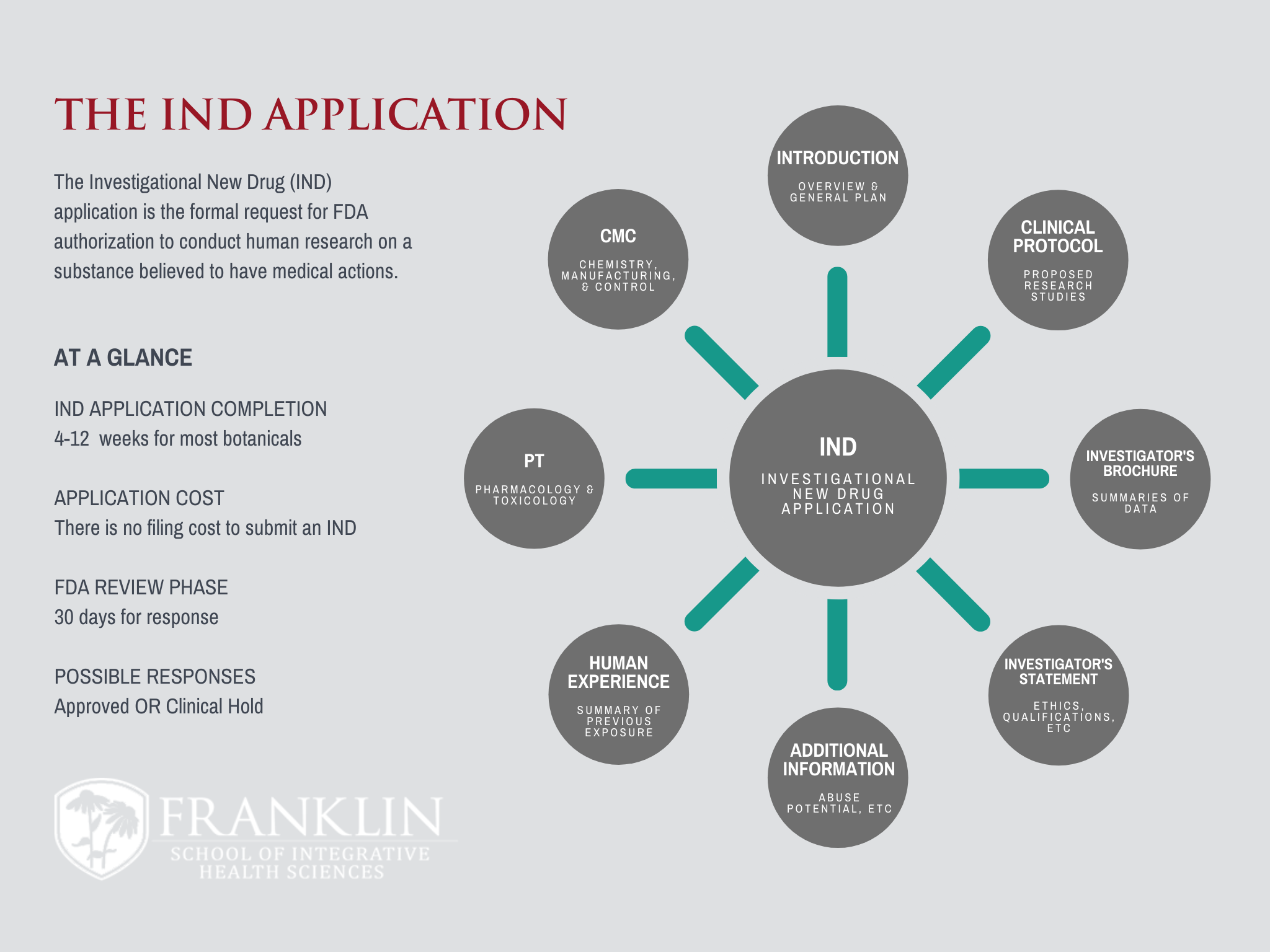
If your study involves a test drug and a medical outcome (i.e. something to prevent, diagnose, or treat disease), the FDA requires an Investigational New Drug (IND) application before your product can be studied in a clinical trial. This application presents evidence to the FDA that your product is relatively safe for use on human subjects and is reasonably expected to offer a benefit.
This is accomplished through a comprehensive and lengthy application which compiles all of the existing evidence on the ingredients, including laboratory tests, animal testing, manufacturing procedures, and history of human use. The IND also requires a proposed research study so that the FDA can confirm its suitability.
The IND is more than a formality; it is a violation of the FDA's Food, Drug, and Cosmetic Act to ship an unapproved drug in interstate commerce (21 U.S.C. § 355(a)). The IND provides authorization test the unapproved new drug in human patients through participating medical centers for the purpose of evaluating the product's safety or efficacy.
Not all products require an IND for clinical research. Natural products, unlike regular drugs, are typically designed to enhance the structure or function of the human body. If this is the case, a clinical trial is still required to substantiate product claims, but the trial can be conducted without an IND.
The key factor here is not the product or formulation; it's the outcome. For drug effects, INDs are required; for health promoting effects, INDs are not required.
A complete IND is a lengthy document, which requires interdisciplinary expertise. Incomplete INDs can result in costly delays to the research process, lengthening the timeline between development and drug approval. Our team of experts can compile portions of the IND or the entire IND application for your proposed studies, depending on your company's needs.

The FDA has a unique, streamlined process for medicinally active plant-based products to gain market authorization. That this process is much faster...

Outcome measures are among the most critical decisions in clinical trial design. After all, this is what determines whether or not your product was...